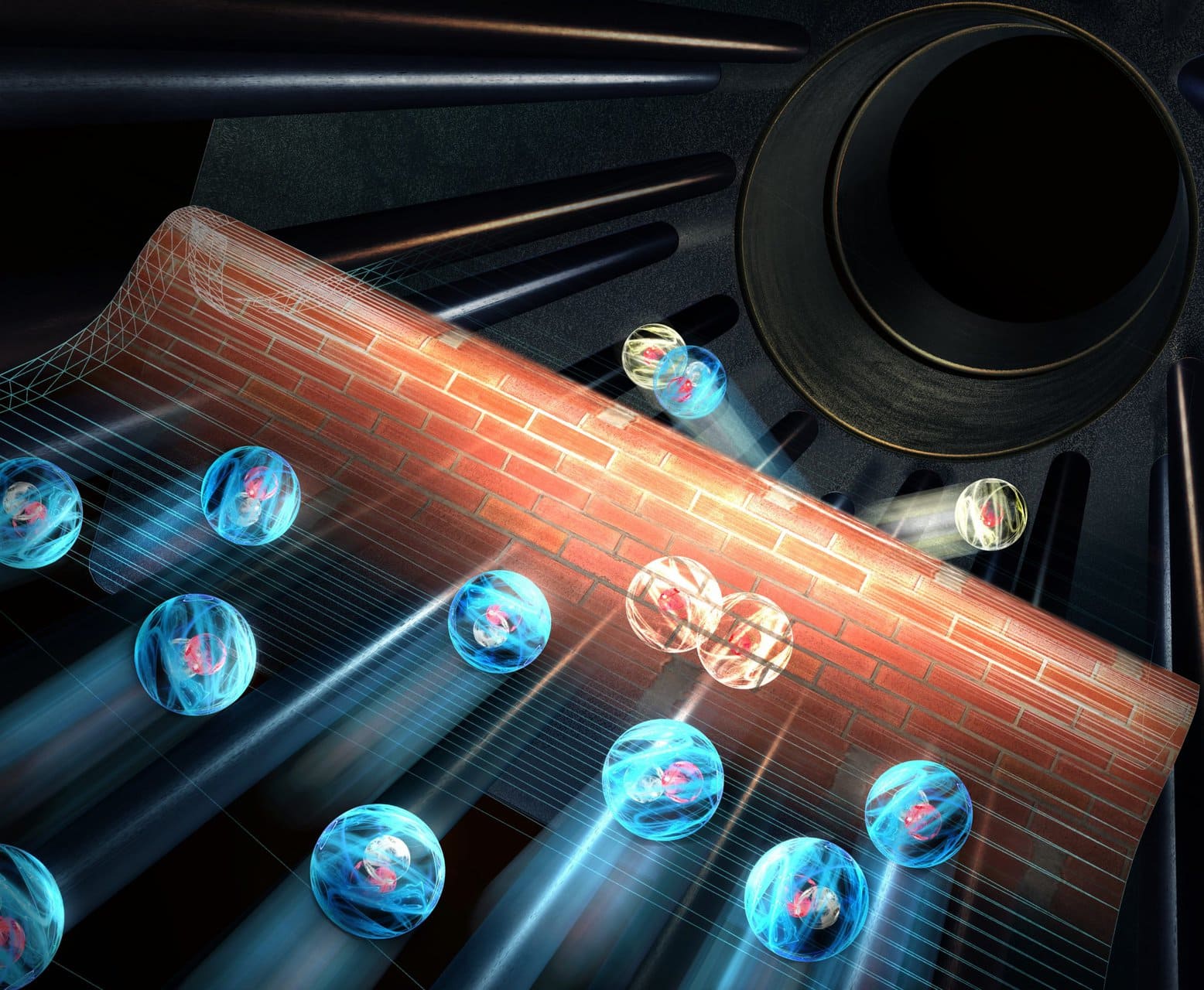
After fifteen years of experiments, a group of researchers has succeeded in substantiating theoretical predictions about the quantum behavior of particles. They observed for the first time the so-called tunnel effect, in which an atom sinks into a molecule with a quantum leap.
Tunnel effect, or Quantum tunnelingIt is an elusive, rare and difficult to predict phenomenon. The laws of quantum mechanics sometimes allow atoms to overcome an energy barrier that is actually too high for them. The impact can sometimes trigger a chemical reaction for which there is not enough energy already.
A research group from Austria has now observed this tunnel effect for the first time in a chemical reaction for which it was also possible to make an accurate calculation. Theorists can breathe a sigh of relief. the results Support their expectations.
Read also
Base math problems on social issues
When mathematics is linked to students’ relevant questions, it can …
Quantum tunnel effect
These predictions are based on quantum mechanical simulations of interactions, which quickly become very complex. If three or four particles – atoms and/or molecules – are involved in an interaction, it is only a matter of time for theorists to calculate when the tunnel effect occurs. But if there are more than four particles involved, the calculations are so complex that a theoretical prediction is almost impossible.
That’s why scientists usually simulate chemical reactions using computer models that largely ignore quantum effects. But is this always right? Do scientists understand quantum effects well enough to build such models?
Experience
To determine this, an experiment is needed in which the tunnel effect occurs, is easily measurable and the interaction is simple enough to accurately calculate quantum mechanical effects.
“During a conversation with a colleague, we came up with the idea for the experiment,” the physicist emails Roland Wester from the University of Innsbruck in Austria. “We chose to react negatively charged deuterium (a heavy form of hydrogen, DS) with hydrogen gas.” Hydrogen molecules consist of two hydrogen atoms.
In the experiment, Wester and his colleagues trapped deuterium and cooled it to -253 degrees Celsius. Then they added hydrogen gas. Normally, the two react with each other to form free, negatively charged hydrogen atoms and molecules composed of deuterium and hydrogen. But at the lower temperatures in the experiment, the deuterium particles don’t have enough energy for this reaction.
The only way deuterium and hydrogen particles can form is through the rare quantum tunneling effect, where deuterium particles can sometimes make a quantum leap. This way they overcome the energy barrier, and they can still interact with hydrogen gas.
15 years of experiences
Such quantum jumps are rare. To determine how often it occurs, the researchers collected several deuterium molecules and hydrogen molecules. Then they waited 15 minutes and looked at how many free, negatively charged hydrogen atoms were formed. This indicates the number of quantum jumps that occurred.

In other words, this experiment seems rather simple: add deuterium and hydrogen gas together, wait 15 minutes, and count the hydrogen atoms. But it took 15 years to build and adjust the experiment to be sensitive enough to measure this. Now this has finally happened. The results confirm quantum mechanical calculations that have been awaiting the outcome of the experiment since 2018.
Wester: ‘Our measurements have shown that the calculations are indeed accurate. This gives confidence that such calculations can be performed for other systems in order to provide a broader view of the role of quantum tunneling in chemistry.

“Thinker. Coffeeaholic. Award-winning gamer. Web trailblazer. Pop culture scholar. Beer guru. Food specialist.”


/s3/static.nrc.nl/images/gn4/stripped/data114435331-287dc4.jpg)


More Stories
Architects cheer in the run-up to the election
OnePlus Watch 2 review: An easy-to-use smartwatch with long battery life
In Van der Poel's absence, Alpesen Desoninck is counting on two hill specialists in Flesch Walloon