Lithium-ion batteries commonly used, for example in smartphones and electric cars, contain a flammable liquid. A newly developed solid can replace this liquid. This could lead to safer and more compact batteries.
Everyday applications in phones, laptops, bicycles and cars require batteries that can provide enough power and can be charged easily and often. Currently, lithium-ion batteries are the best option. It is light and effective. But they are not perfect. The liquid conducting the charged particles is now flammable and toxic.
Replacing the liquid with a solid would make the batteries safer. But it is not easy to find a solid with similar performance to the current liquid. Chemists from the University of Liverpool in the UK now have it Promising new material They are found with the help of a computer program based on artificial intelligence (AI).
Read also
Chemist Siegbrin Otto wants to create life in the laboratory from a dead chemical soup, and he's already on his way
Chemist Siegbrin Otto is working on a miracle: he is trying to grow life from a lifeless chemical soup in his laboratory in Groningen. Recently received…
Liquid or solid batteries
Batteries consist of a negatively charged electrode (anode) and a positively charged electrode (cathode). There is an electrolyte between them. When a battery is connected, positively charged particles (ions) flow through the electrolyte, from positive electrode to negative electrode, while electrons flow through the connected device. This is how the battery supplies power. When the battery is charged, the ions return to the positive electrode.
In lithium-ion batteries, the electrolyte through which the lithium ions move is a liquid substance. Because this liquid is flammable, lithium-ion batteries must be properly filled.
“By using a solid as the electrolyte, this risk no longer exists,” the researchers say. “Using a solid electrolyte would also make it possible to replace the current graphite anode with an anode made of lithium metal.”
The lithium metal anode ensures higher energy density and therefore better batteries. Lithium minerals and liquid electrolytes don't mix. If you combine them, a short circuit can easily occur, and sometimes a fire can occur. Solid electrolytes do not have this problem.
New materials
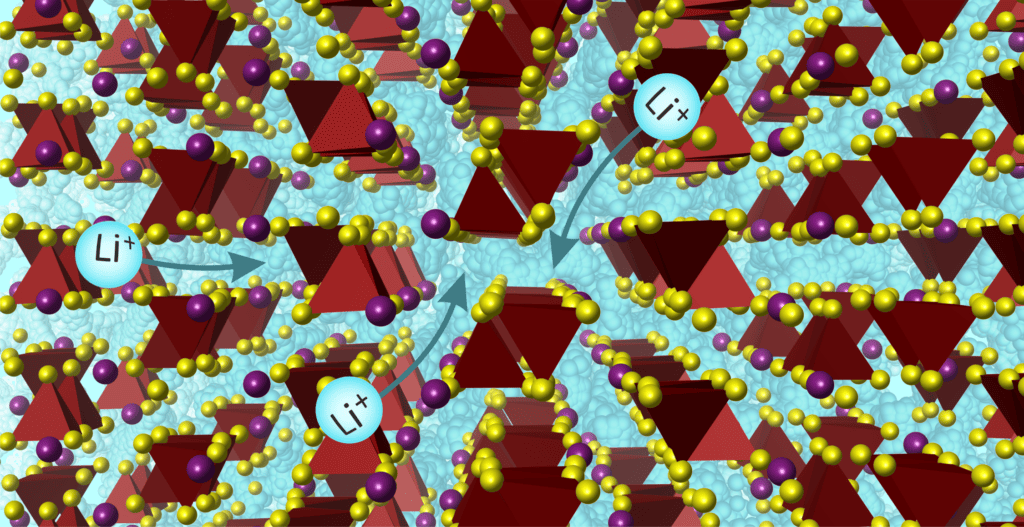
Despite much research, no solid electrolyte is yet known that can conduct ions as well (or better) than liquid ones. New materials can do that.
The substance, with the chemical name Lee7bad2s7Researchers have developed this with a little help from artificial intelligence. They designed the program materials and controlled the process with their own insights and background knowledge.
With the new solid, not only is the chemical composition important, but also the structure in which the atoms are arranged. This atomic structure, combined with composition, ensures that the material conducts ions better than other solids.
This development enables batteries that are safer and require less leakage protection. This means they can be more compact than current batteries.
Researchers confirm that so far this material has only been manufactured and tested in the laboratory on a small scale. Future research should show whether it is stable enough to be charged repeatedly. The question also remains whether these things can be produced on a large scale for commercial applications such as phones and cars.

“Total coffee specialist. Hardcore reader. Incurable music scholar. Web guru. Freelance troublemaker. Problem solver. Travel trailblazer.”









More Stories
Brabanders are concerned about climate change.
The “term-linked contract” saves space on the electricity grid.
The oystercatcher, the “unlucky national bird,” is increasingly breeding on rooftops.